| |
 |
 |
|
 |
| |
Developments of sustainable materials and processing based on electrochemistry and thermodynamics
|
|
| |
Our laboratory was inaugurated in 1994 during the reorganization of Department of Materials Science and Engineering. The forerunner of our laboratory was the Electrometallurgy Division of the Department of Metallurgy, where we were working on hydrometallurgy, e.g. aqueous thermodynamics, electrowinning, leaching and extraction. Our group is currently interested in various studies based on electrochemistry and thermodynamics, such as new smelting and recycling processes for rare metals and intermediate-temperature fuel cells with proton-conducting electrolytes.
|
|
| |
|
|
| |
|
|
| |
Academic staff |
|
| |
| |
|
|
| |
 |
| Professor : Uda, Tetsuya |
| |
| Research Topics |
| Current focus is technology and science for
intermideate temperature fuel cell which can be operated from 200 °C to
500 °C. Especially, my strong interest is electrochemistry and
thermochemistry of fuel cell using solid phosphate and proton
conducting oxide as electrolyte. |
| |
| Contact / Office |
Room 618, School of
Engineering Science Bldg, Yoshida Campus
TEL +81-75-753-5439 / FAX +81-75-753-5284
uda.tetsuya.5e kyoto-u.ac.jp kyoto-u.ac.jp |
|
| |
|
|
|
|
| |
|
|
| |
| |
|
|
| |
 |
| Associate Professor : Toyoura, Kazuaki |
| |
| Research Topics |
・ Development of analysis method on atomic diffusion and ionic conduction in solids
・ Systematic analyses on various proton- conducting oxides
|
| |
| Contact / Office |
Room 630, School of
Engineering Science Bldg, Yoshida Campus
TEL +81-75-753-3551 / FAX +81-75-753-5284
toyoura.kazuaki.5r kyoto-u.ac.jp kyoto-u.ac.jp |
|
| |
|
|
|
|
| |
|
|
| |
| |
|
|
| |
 |
| Assistant Professor : Hatada, Naoyuki |
| |
| Research Topics |
| Development of new inorganic electrolytes and
electrodes for high-performance fuel cells, and relevant materials
processing techniques. |
| |
| Contact / Office |
Room 618, School of
Engineering Science Bldg, Yoshida Campus
TEL +81-75-753-5445 / FAX +81-75-753-5284
hatada.naoyuki.8u kyoto-u.ac.jp kyoto-u.ac.jp |
|
| |
|
|
|
|
| |
|
|
| |
| |
|
|
| |
 |
| Assistant Professor : Kishimoto, Akihiro |
| |
| Research Topics |
| Development of smelting and recycling processes for metals. |
| |
| Contact / Office |
Room 630, School of
Engineering Science Bldg, Yoshida Campus
TEL +81-75-753-3551 / FAX +81-75-753-5284
kishimoto.akihiro.5m kyoto-u.ac.jp kyoto-u.ac.jp |
|
| |
|
|
|
|
| |
|
|
| |
|
|
| |
 |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
New smelting processes for titanium
|
|
| |
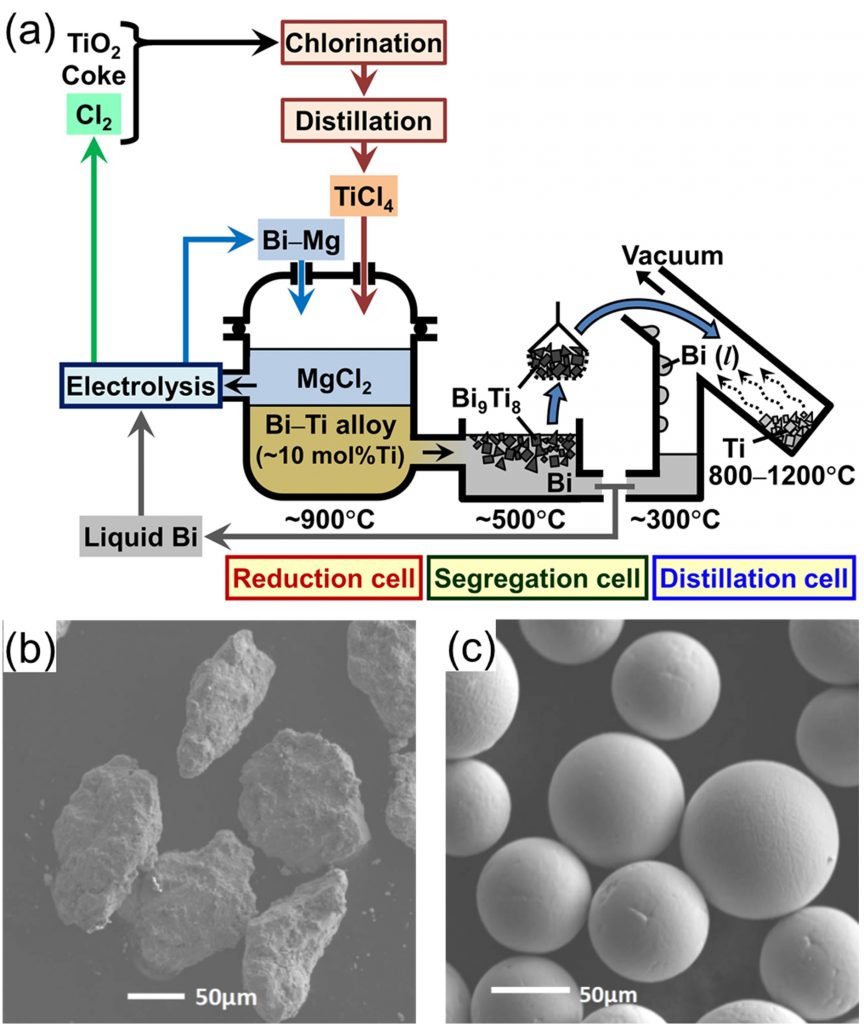
Titanium (Ti) has high specific strength, high corrosion resistance, and abundant resource. However, use of Ti is limited because costs for smelting, melting, and working Ti are higher than that of steel or aluminum. From this background, we investigate new processes for Ti.
1) New smelting process for Ti via. Bi-Ti alloy
Titanium tetrachloride (TiCl4) is reduced into liquid bismuth (Bi) by magnesium (Mg) to form Bi-Ti liquid alloy at 1173 K. In the next step, Ti-rich compound, Bi9Ti8, is recovered by cooling the alloy to 773 K, and pure Ti is separated from Bi by vacuum distillation. In this process, the production speed is expected to be faster than the current commercial process, and Ti powder can be produced.
2) Direct production process for Ti sheet by electroplating in molten salt
Electrodeposition of Ti is carried out in TiCl2-added molten halides, such as magnesium dichloride, sodium chloride, and potassium chloride, at high temperature in the new process. Ti is flatly and smoothly deposited by the electrolysis and peeled off from cathode substrate. Our group demonstrated flat electrodeposition of Ti with simple equipment and peeling off Ti sheet from substrate.
|
|
| |
|
|
|
|
| |
|
|
| |
|
|
| |
|
|
| |
Protonic ceramic fuel cells (PCFCs)
|
|
| |
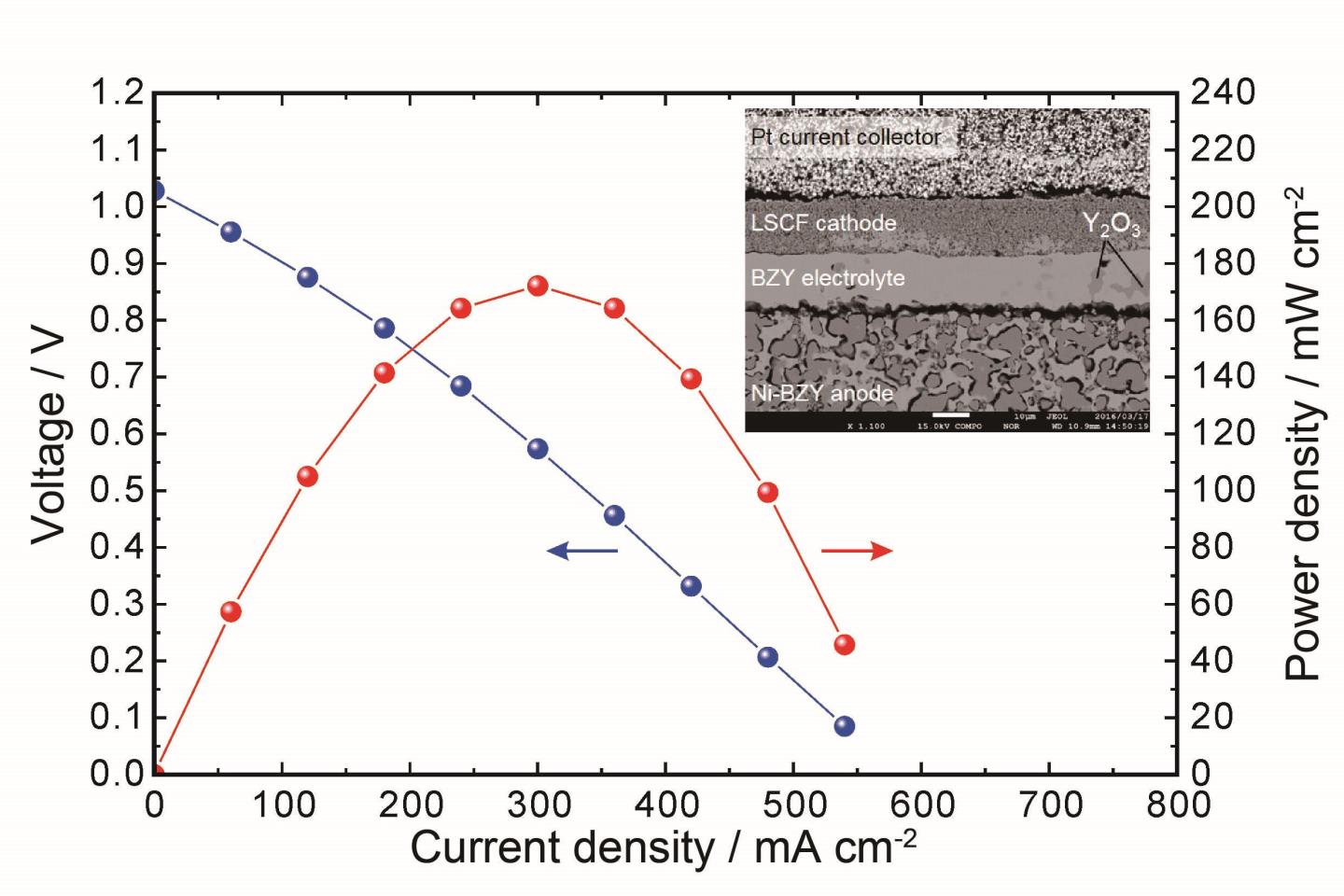
Because of potential application as efficient power generation devices, protonic ceramic fuel cells (PCFCs) operating in the intermediate temperature range (400-600 °C) are receiving increasing attention. We have developed PCFCs using yttrium-doped barium zirconate (Y-doped BaZrO3, BZY) with both high proton conductivity and excellent chemical stability, which is regarded as the most promising candidate as the electrolytes in PCFCs. In our studies, the ionic conductivity of BZY has been enhanced up to 30 mS/cm at 600 °C. In addition, we have succeeded in fabricating an anode-supported cell with high performance (open-circuit voltage: 1.03 V, peak power density: 172 mW/cm2).
|
|
| |
|
|
| |
|
|
| |
|
|
| |
Thermochemical energy storage materials
|
|
| |
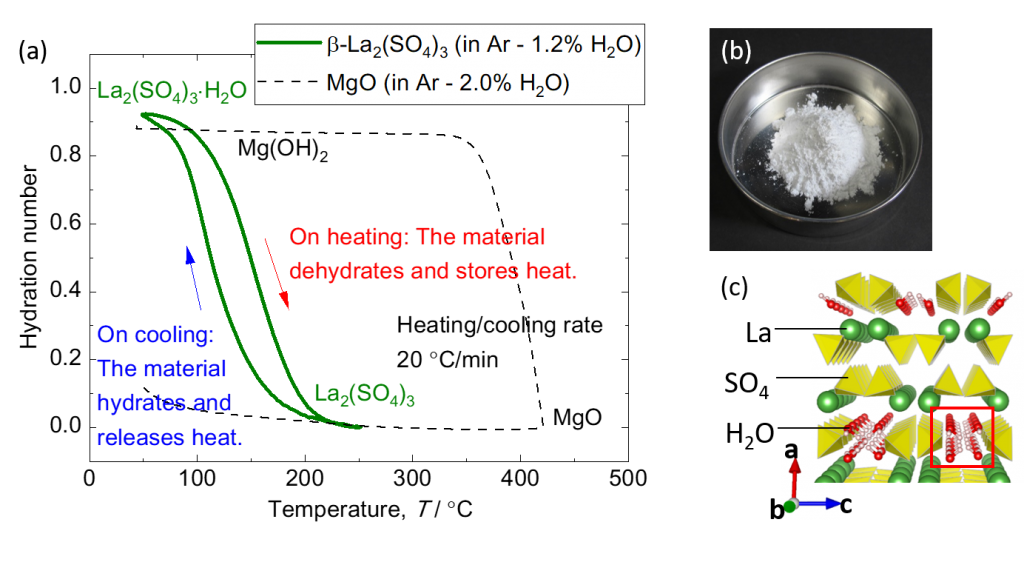
Factories and power plants emit large amounts of waste heat at low temperatures below 250 °C. The use of fossil fuels can be reduced if waste heat can be stored using thermal energy storage technology and used effectively where and when it is needed. Therefore, we are focusing on thermal energy storage technology that uses heat from chemical reactions. To achieve this, it is necessary to find a chemical reaction with a reaction temperature suitable for the waste heat, a high reaction rate, and sufficient heat storage density. We have found for the first time that the hydration and dehydration reactions of rare-earth sulfates proceed rapidly in the temperature range of 100-250 °C. Experiments and computer simulations have shown that the dehydration and hydration reaction of this material is driven by the insertion/desertion of water molecules into the crystal structure. We are now investigating the mechanism of this reaction in detail, and are aiming to develop new thermochemical energy storage materials based on the knowledge obtained.
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
Efficient PES mapping for characterizing ionic conductivity
|
|
| |
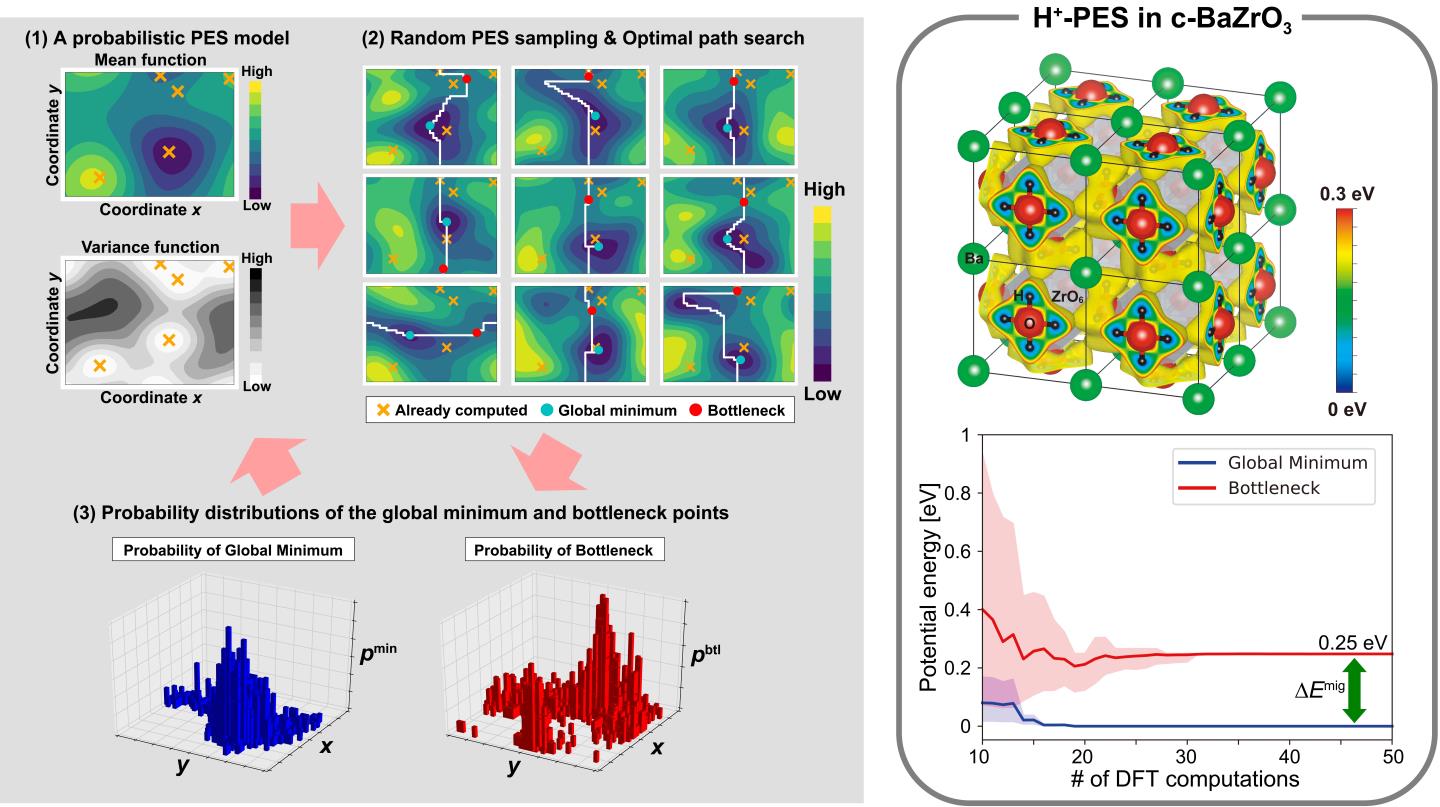
The mobility of an ion in a host crystal is characterized by the entire potential energy surface (PES) of the mobile ion. Although the entire PES can be theoretically evaluated by exhaustive local structural optimizations around the mobile ion, it requires huge computational costs, particularly by first-principles calculations. We have therefore developed a machine-learning (ML) method of efficient PES mapping with several collaborators, in which only the global minimum point and the bottleneck point on the optimal path are evaluated selectively.
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
 |
|
|